FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology
The journal is the first and most reputable in Russia and EurAsEC (Eurasian Economic Community) countries peer-reviewed periodical that publishes materials on new medical technologies, economic optimization of drug therapy, quality-of-life and healthcare problems.
The journal was founded in 2008.
The impact factor of this journal, as shown in the Russian Science Citation Index (RSCI) is the highest among the periodicals in the areas of pharmacoeconomics, health technology assessment, and epidemiology. According to RSCI, the biennial impact factor (without self-citations) was 0.325 in 2013, 0.411 in 2014, and 0.722 in 2015.
The journal publishes various materials on pharmacoeconomics and pharmaco-epidemiology including the methodology, data analysis and results of studies on public health, medical technologies and economic aspects of drug therapies. The original articles and literature reviews cover Cost-of-Illness Analysis, Cost-Minimization Analysis, Cost-Effectiveness Analysis (CEA), Cost-Utility Analysis (CUA), Cost-Benefit Analysis (CBA), Quality of Life Assessment (QoL), Patients' Preferences & Patients’ Satisfaction indices and related topics.
Our aims and priorities focus on scientific and information support to the decision-makers and experts in public drug supply, health providers, research and education professionals, as well as pharmaceutic and insurance companies.
Languages: Russian, English
Periodicity: 4 issues per year (quarterly).
Copies of this journal are distributed under the Creative Commons Attribution 4.0 License: full-text materials are freely available to the public in an open access repository.
Distribution of the printed version: Russia, the EurAsian Economic Community countries (Belarus, Kazakhstan, Kyrgyzstan, Tajikistan, Uzbekistan, Armenia, Moldova)
The editorial board of “FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology” includes leading experts in pharmaco-economics, clinical pharmacology, medical technology assessment, epidemiology, and public health from Russia, USA and Spain.
The editorial board maintains the policy of full compliance with all principles of publishing ethics. Our ethical standards and codes conform to those of top international science publishers.
All submitted materials undergo a mandatory double-blind peer review.
Media Certificate of Registration: ПИ №ФС77-32713 of August 01, 2008.
ISSN 2070-4909 (Print)
ISSN 2070-4933 (Online)
By the decision of the Higher Attestation Commission (HAC) of Russia, “FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology” is included in the "List of top peer-reviewed scientific journals and publications" where scientists seeking academic degrees are required to publish their results.
The journal appears in the Russian Universal Scientific Electronic Library (RUNEB) elibrary.ru and is also present in the database of the Russian Science Citation Index (RSCI). Concise versions of major articles from this journal are published by the All-Russian Institute for Scientific and Technical Information (VINITI). The journal is also indexed by "Ulrich's periodicals Directory" – a global information system of periodicals and continued publications.
Current issue
ORIGINAL ARTICLES
What is already known about thе subject?
► The use of machine learning (ML) and scenario modeling (SM) methods in medicine and insurance enables to develop effective and explicable algorithms for simulating processes and analyzing their outcomes
► Developing and integrating ML models into modern medical information systems optimize routine processes in healthcare organizations
► Combining SM with ML enables more accurate modeling of complex dependencies and getting results based on individual patient and physi-
cian characteristics that promotes medical and pharmaceutical care for children
What are the new findings?
► The approaches to preliminary data processing on drug prescriptions for children, development of additional features based on the data analysis from the research information base, as well as adaptation of the obtained results into a form convenient for interpretation by ML models were described in detail
► Drug prescribtion process was formulated as a task of multi-label classification for ML models; their mathematical substantiation proposed a new metric to evaluate model accuracy, measuring the proportion of true labels among the most probable predictions
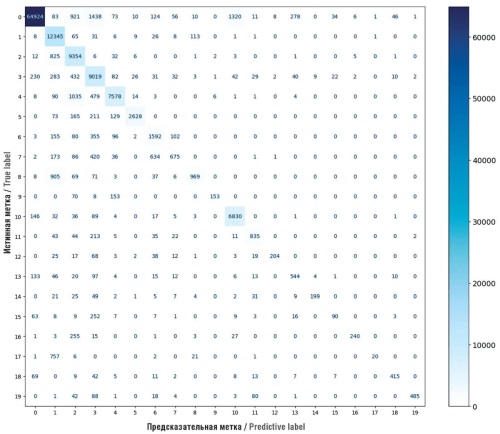
► Comparative analysis 25 ML models and neural networks allowed us to determine eXtreme gradient boosting classifier (XGBC) as a most effective model for multi-label classification of active drug substances, achieving 85% accuracy
How might it impact the clinical practice in the foreseeable future?
► Combined system of SM of pharmaceutical care for children with the XGBC model enables to get accurate predictions of drug prescriptions, which can be used for estimating pharmacotherapy costs upon developing individual drug insurance programs
► The obtained model can help to create an assortment of stock ready-made dosage forms for pharmacies, providing a list of drugs in demand in pediatric practice, and improving availability of pharmaceutical care
► Integrating the model as a clinical decision support system promotes personalization and improvement of medical care quality by enabling the physician to involve predicted values based on a mathematical interpretation of the patient individual characteristics
Objective: determining the most appropriate machine learning method to solve the problem of drug prescribtion for children, evaluating its performance and potential for implementation into scenario modeling systems of the pharmaceutical care structure.
Material and methods. The study was based on data on drug prescription for children from medical information systems of Moscow clinics for the period from January to December 2023 including information about patients, the date of treatment, diagnoses, prescribed medications and the doctor's specialty. Preliminary data processing enabled to extract additional features and define the process as a multi-label classification task. The following model architectures were developed and validated: fully connected neural network (FCNN), convolutional neural network (CNN), One-vs-Rest (OvR) classifier, eXtreme gradient boosting classifier (XGBC), and RandomForestClassifier (RFC). The models were evaluated using area under curve (AUC) of receiver operating characteristic (ROC), F1-measure metrics and Custom Accuracy metrics.
Results. The XGBC model showed the best results for all tasks and metrics. After optimizing the model and dataset, the AUC ROC reached 0.9993, the F1-measure was 0.8318, and Custom Accuracy metric was 0.8548. The model effectively predicted the prescription of drugs with similar pharmacological effects, allowing us to evaluate the structure of pharmaceutical care within a specific scenario. Optimization of the data and model increased the accuracy of predictions up to 85%.
Conclusion. The XGBC model proved to be the most appropriate for solving the problem of scenario modeling of drug prescribtion. The identified problems with predicting similar drugs validate the demand for further improvement of the model and data. Concurrently, the results obtained attest the potential of integrating machine learning methods into scenario modeling systems for pharmaceutical care.
What is already known about thе subject?
► According to the legislation, the provision of tablet-based medications obtained through the Compulsory Health Insurance (CHI) is to be carried out exclusively in a day hospital setting. Home administration of these medications is only possible after they have been obtained through preferential funding, at the primarily regional level
► Administration of oral anticancer drugs (ACDs) to patients for outpatient use leads to frequent legal disputes between day hospitals and territorial CHI funds
What are the new findings?
► The dynamics in the use of all forms of drug therapy for oncology patients in both inpatient and day hospital settings over a 5-year period was studied
► The findings revealed an annual increase in the use of all forms of ACD therapy in day hospital settings. The use of oral ACDs in these settings exhibited the highest growth rate, suggesting a decreasing reliance on regional funding for the pharmaceutical provision for oncology patients
How might it impact the clinical practice in the foreseeable future?
► The increased use of tablet-based therapy in day hospitals perpetuates existing barriers for patient access to oral ACDs in outpatient settings, giving rise to new legal disputes related to the use of such drugs outside the hospital
► The study highlights the necessity for strategic transformation in the pharmaceutical provision for oncology patients, which could involve either increase in funding from regional budgets at the outpatient stage or the establishment of a unified source of funding
Background. Pharmaceutical provision for oncology patients involves complex and controversial issues, particularly the use of oral anticancer drugs (ACDs) in day hospitals. These facilities often distribute oral medications for outpatient use, leading to frequent legal disputes with territorial Compulsory Health Insurance funds. To determine whether this practice changes over time, it is necessary to analyze trends in the use of ACDs.
Objective: to identify trends in the use of different forms of ACDs in both inpatient and day hospital settings over a 5-year period.
Material and methods. The study analyzed medical care provided in inpatient and day hospital settings using depersonalized hospitalization records from oncology-focused medical organizations. The data from 2019 to 2023 were categorized into three groups: regimens with only oral administration, including combinations of multiple oral drugs; regimens with only injectable forms, including intravenous and subcutaneous administration; and combined regimens, including oral and other forms of administration. The annual distribution and trends in the use of different forms of ACDs were analyzed for both inpatient and day hospital settings over the 5-year period. A time series analysis was carried out to assess the dynamics, including the calculation of absolute and relative growth (or decline) rates. Time series smoothing was performed using aggregation and moving average methods.
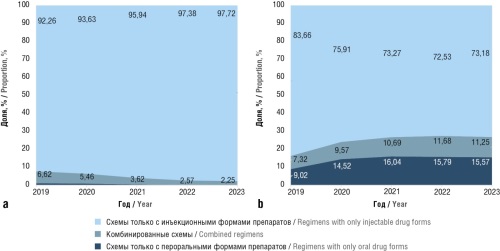
Results. From 2019 to 2023, day hospitals in Russia exhibited an increase in the use of all ACD forms. The highest growth rate (79.9%) was observed for the use of oral therapy in 2020, with an overall increase from 9.25% to 29.09% over the study period. The combined regimens used in day hospitals demonstrated an increase in the proportion of oral therapy from 10.54% to 29.46%. However, inpatient settings exhibited a rise in the use of only injectable regimens from 17.65% to 23.32%. Conversely, the role of inpatient oral therapy significantly declined, with the proportion of oral-only and combined regimens decreasing from 43.59% to 1.18% and 30.47% to 12.95%, respectively.
Conclusion. The results provide an evaluation of the trends in ACDs use in Russian hospitals. Despite an overall increase in the use of all ACD forms in day hospitals, oral medications exhibited the highest growth rate.
What is already known about thе subject?
► The key parameter for evaluating the effectiveness of the therapy is the achievement of the target level of glycated hemoglobin (HbA1c) <7%
► Currently, glucagon-like peptide type 1 receptor agonists (GLP1 RAs) are widely used, however, therapy with drugs of this group is expensive
► The alogliptin and pioglitazone combination, belonging to the groups of dipeptidyl peptidase-4 and thiazolidinedione inhibitors, also shows high efficacy when achieving the target level of HbA1c<7%
What are the new findings?
► Using alogliptin and pioglitazone combination for type 2 diabetes mellitus patients can significantly reduce the total costs associated with applying hypoglycemic drugs and the treatment of concomitant cardiovascular complications in comparison with GLP1 RAs while maintaining the efficacy of therapy
How might it impact the clinical practice in the foreseeable future?
► Thorough assessing patients’ profiles enables the optimal hypoglycemic therapy option to be chosen, which will significantly reduce drug costs when achieving the effectiveness targets
► The saved funds will allow providing therapy to an additional number of patients with type 2 diabetes mellitus
Objective: to comparatively assess the weighted average costs associated with the use of alogliptin-pioglitazone combination versus glucagon-like peptide-1 receptor agonists (GLP-1 RAs).
Material and methods. A retrospective study involved pharmacoeconomic analysis, including cost-minimization analysis and budget impact analysis, with consideration to the efficacy of the compared therapeutic regimens. Data regarding treatment regimens and safety profiles of the alternatives were obtained from the published results of phase III clinical trials and clinical guidelines. Drug pricing information was derived from the state registry of maximum retail prices and auction results.
Results. According to the budget impact analysis, the fixed-dose combination of alogliptin and pioglitazone in the treatment of patients with type 2 diabetes significantly reduces the total costs associated with the administration of hypoglycemic agents and management of concomitant cardiovascular complications. A complete replacement of GLP-1 RAs with the alogliptin-pioglitazone combination in the treatment of 83,582 patients is expected to reduce costs by 42.1% (or by 10,934.5 million rubles) over 3 years. The savings generated from this replacement will enable alogliptin-pioglitazone combination therapy for additional 18,622 patients with type 2 diabetes in the first year and 21,103 patients in subsequent years.
Conclusion. The use of alogliptin-pioglitazone combination as an alternative to GLP-1 RAs appears economically justified and reasonable with respect to reducing costs associated with hypoglycemic therapy and treatment of cardiovascular events.
What is already known about thе subject?
► According to a network meta-analysis, netakimab therapy was significantly superior or demonstrated a trend towards higher efficacy compared to other biological disease-modifying antirheumatic drugs (bDMARDs) by the ASAS20/40 and BASDAI50 criteria
► Netakimab showed higher clinical and economic effectiveness in the treatment of ankylosing spondylitis compared to other bDMARDs from the class of interleukin-17 inhibitors – ixekizumab and secukinumab
What are the new findings?
► The costs for additional effect provided by netakimab therapy according to ASAS20, ASAS40 and BASDAI50 criteria are lower compared to other bDMARDs by 31–77%, 34–80%, and 20–69%, respectively
How might it impact the clinical practice in the foreseeable future?
► The expansion of using netakimab in ankylosing spondylitis treatment is expected to enhance therapeutic options and improve patient prognosis
Objective: to evaluate clinical and economic effectiveness of netakimab therapy for ankylosing spondylitis (AS) in comparison with other biological disease-modifying antirheumatic drugs (bDMARDs) currently employed in clinical practice in the Russian Federation.
Material and methods. The evaluation of clinical and economic effectiveness was carried out from the perspective of the healthcare system based on the results of a network meta-analysis of randomized clinical trials. Clinical effectiveness criteria included indicators of therapeutic response according to the Assessment of Spondyloarthritis International Society criteria, specifically ASAS20 and ASAS40, as well as by the Bath Ankylosing Spondylitis Disease Activity Index BASDAI50. A time horizon of the study was set at 2 years. Costs of pharmaceuticals were calculated according to the registered maximum retail prices, inclusive of value-added tax. In case of availability of biosimilars on the market, calculations were based on the minimum values of the registered prices.
Results. According to the network meta-analysis, the efficacy of netakimab significantly surpassed that of other bDMARDs or demonstrated a trend towards higher efficacy for AS. The costs per patient achieving an additional response compared to standard therapy without bDMARDs when treated with netakimab appeared 31–77%, 34–80%, and 20–69% lower than those of other bDMARDs, according to ASAS20, ASAS40, and BASDAI50 criteria, respectively.
Conclusion. Netakimab exhibits a higher clinical and economic effectiveness compared to other bDMARDs and may be considered as the preferred therapeutic option in comparable clinical scenarios for active AS, thereby ensuring cost-effectiveness for the healthcare system along with achieving the efficacy criteria compared to the standard treatment regimen. The limitations of the analysis determine the need for further research in this area.
What is already known about thе subject?
► Anesthetic support constitutes a key and often significant cost element in calculating the expenses of providing medical care within the context of medical services or cases involving the use of anesthesia
► Сurrently, there is no universally accepted classification of anesthesia methods, and the existing medical services nomenclature lacks sufficient anesthesia methods to serve as a basis for calculations
► A standardized list of anesthesia methods, along with its structural and financial detailing, has not been previously presented
What are the new findings?
► A unified classification (list) of anesthetic management methods aimed at assessing financial costs was developed
► Each anesthesia method in the classification was enriched with structural and financial components in the form of protocols, allowing for transparent and objective use as a basis for calculations
How might it impact the clinical practice in the foreseeable future?
► The developed protocols for each anesthesia method can be applied in future calculations when updating and forming new services and methods for diagnosis-related groups and high-tech medical care that include anesthesia
Background. The costs of anesthetic support represent a significant and essential component in the expense structure of medical services, diagnosis-related groups (DRGs), and high-tech medical care (HMC) that involve anesthesia. Consequently, there is a need to develop a unified methodology for calculating such costs, which ensures transparency and objectivity in the expense structure data. Developing appropriate protocols for each anesthesia method will enable their use in future calculations when updating and forming new medical services, DRG methods, and HMC that incorporate anesthesia.
Objective: to standardize approaches to calculating costs of anesthetic support.
Material and methods. The study was conducted in two stages. In the first stage, a classification (list) of anesthesia methods was developed for use in calculating the financial costs of anesthetic management. In the second stage, the structural and financial detailing of protocols for anesthesia methods was carried out in accordance with the classification. At each stage, the work of specialists from the Center for Healthcare Quality Assessment and Control was validated by external experts, including anesthesiologists and intensivists.
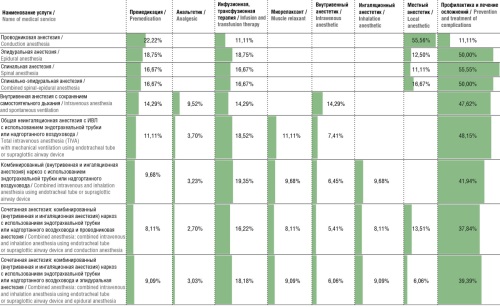
Results. The developed classification of anesthesia methods for calculating financial costs of anesthetic management, validated by external experts, included nine main methods. For each method, protocols were created for the first hour, the second, and each subsequent hour. These protocols facilitated the calculation of direct costs associated with providing anesthetic management, including labor costs for personnel directly involved in the medical service, costs of consumables, pharmaceuticals, depreciation, and indirect costs excluding the payroll of additional staff. The largest share of costs was attributed to consumables (over 50% for each anesthesia method). Additionally, calculations were made for indirect costs necessary to support the overall activities of medical organization.
Conclusion. The developed protocols for each anesthesia method can be applied in future calculations when updating and forming new services and methods for DRGs and HMC that include anesthesia.
What is already known about thе subject?
► There is a regional variation in the economic accessibility and range availability of medicines
► The pandemic had an impact on the range availability of medicines
What are the new findings?
► It was shown that regional variations in the economic accessibility and range availability of medicines may differ within one group of drugs for different trade names
► It was established that the dynamics of prices were influenced by various external shocks, which had different effects on the economic accessibility of domestic and foreign-produced drugs
► It was shown that in addition to regional variation prices experience spatial autocorrelation
How might it impact the clinical practice in the foreseeable future?
► Given the different economic accessibility and range availability of medicines, regional differentiation of treatment tactics and selection of medicines may also increase
Background. In the Russian Federation (RF), the problems of supplying the population with medicines are associated with both their physical and economic accessibility, which are heavily dependent on such various external shocks as pandemic, special military operation and other ones. There is a regional differentiation in the availability of individual drugs and their price levels, which determines the variability of market responses to external shocks and government-developed mechanisms for stabilizing markets.
Objective: to reveal regional availability dependencies of antitussive drugs and expectorants.
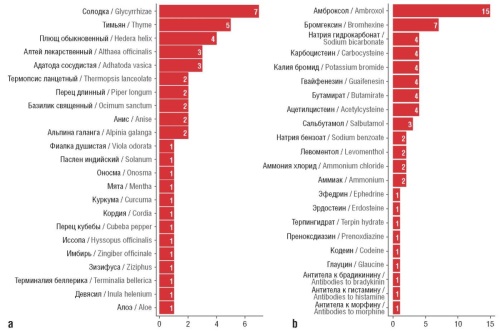
Material and methods. The study was carried out using data from the online retail sales analytics service in the RF, which forms a database based on cash receipts containing information on the sales price of 55 drugs for the period from January 1, 2021 until December 31, 2023 in the context of 83 RF constituent entities. Analysis included assessment of the structure of medicines sold by their composition, place of production, inclusion in the list of vital and essential drugs, as well as dynamics, variations and spatial autocorrelation of prices, availability of medicines in the regions of RF.
Results. Range availability increased for 35 out of 55 drugs under review while all of them in 2023 were represented only in 5 RF constituent entities. Prices were characterized by high variation both in trade names (TNs) and RF constituent entities, varying from 13% (Doktor MOM®) to 181% (Kuka). The dynamics of prices for domestic and foreign produced drugs until 2022 and after this timepoint differed. The economic availability of medicines in terms of TNs and RF constituent entities was in a very wide range (from 0.003 to 1.4 daily median income). These differences were not chaotic, but spatially dependent: positive spatial autocorrelation of prices was established.
Conclusion. The analysis revealed different economic accessibility and range availability of drugs in regions where external shocks have a significant influence on their dynamics. At the same time, we observed the variability of the restoration rate of spatial equilibrium of drug markets in terms of TNs, that should be considered when designing stabilization activities.
What is already known about thе subject?
► Human placenta hydrolysates (HPH) are used in hepatology for the treatment of steatohepatitis (alcoholic, metabolic and mixed etiology)
► The results of experimental and clinical fundamental studies indicate the effectiveness of the prospects for using HPH for the treatment of fatty liver disease with iron overload
What are the new findings?
► For the first time, the HPH effect on iron removal from liver whith simultaneous preventing damage to kidneys, brain, and myocardium in the proposed iron overload metabolic-associated fatty liver disease (МAFLD) rat model was studied
► Complex changes in biomarkers of liver, kidney, hematopoiesis, inflammation and thrombus formation that occur during the model reproduction were characterized
► The efficacy and safety of using standardized HPH in the treatment of experimental iron overload МAFLD were shown
How might it impact the clinical practice in the foreseeable future?
► Standardized HPH is a very promising direction in the search for effective and safe hepatoprotectors for the treatment of MAFLD. However, additional studies are required in clinical settings to confirm these results
Background. The combination of metabolic-associated fatty liver disease (МAFLD) with iron overload occurs in approximately 1/3 of patients and is extremely difficult to treat. In addition to the fact that no specific treatments have been developed for this МAFLD form, there are also few experimental models on which such agents could be tested.
Objective: to create a MAFLD model and to study the effectiveness of using human placenta hydrolyzate (HPH) in experiment.
Material and methods. In experiment, the rats were divided into three groups: Group 1 was on a normal diet and drinking water, in Groups 2 and 3, a model of liver iron overload was reproduced by intraperitoneal administration of iron sulfate for 12 days under conditions of adding saturated fats (palm oil) and fructose to the diet. On Day 13 of the study, blood was collected from animals in Groups 1 and 2 for biochemical testing and autopsy material (liver, kidneys, brain, heart) for histopathological examination. In Group 3, standardized HPH was administered in a therapeutic dose intramuscularly for 4 weeks. On Day 41, blood and autopsy material were collected. The model was used to test the effectiveness of using standardized HPH and to characterize complex changes in more than 20 biomarkers of liver and kidney functions, hematopoiesis, inflammation, and thrombus formation.
Results. HPH injections were shown to be an effective treatment for iron overload МAFLD. Specifically, after reproducing the model on Day 41, levels of ferritin (intact: 201±45 μg/l; model: 254±12 μg/l; p<0.0001), aspartate aminotransferase (AST) (intact: 114.9±27.3 U/l; model: 301,3±30,3 U/l; р<0.000001), alanine aminotransferase (ALT) (intact: 22.8±3.2 U/l; model: 58.7±5.5 U/l; p<0.00014), leukocytes (intact: 4.6±1.3×109 cells/l; model: 6.9±0.8×109 cells/l; p<0.01), thrombocytes (intact: 509.7±121.6×109 cells/l; model: 820.2±50.5×109 cells/l; p<0.01) increased reliably. Total protein levels (intact: 46.2±2 4.6 g/l; model: 45.5±5.8 g/l; p=0.002), serum creatinine (intact: 35.7±1.2 μmol/l; model: 23.3±1.4 μmol/l; p<0.00001) and glomerular filtration rate (GFR) (intact: 169±5 ml/min/1.73 m2; model: 154.1±7.1 ml/min/1.73 m2; p=0.04) decreased. HPH administration resulted in normalization of the listed indicators of polyorgan pathology on Day 41: reliable reduction of ferritin (141±24 μg/l; p<0.001), AST (166.7±51.3 U/l; p=0.00±77), ALT (36.4±7.2 U/l; p=0.00001), leukocytes (4.5±2.7×109 cells/l; p=0.039), thrombocytes (639.0±92.3×109 cells/l; p=0.00157) and reliable elevation of total protein (55.9±3.8 g/l; p=0.0014), normalization of creatinine (27.7±1.5 μmol/l; p=0.0002), and GFR (169.8±6.2 ml/min/1.73 m2; p=0.0011). Histological analysis revealed that HPH promoted hepatic iron excretion while preventing renal, brain, and myocardial damage in the proposed iron-overload МAFLD model.
Conclusion. The administration of standardized HPH is effective and safe in the therapy of experimental iron overload МAFLD and prevents polyorgan pathology.
What is already known about thе subject?
► Interleukin inhibitors, including netakimab, an interleukin-17 inhibitor, are drugs characterized by high clinical efficacy in psoriatic arthritis (PsA)
► According to a network meta-analysis, netakimab therapy was superior or showed a trend towards higher efficacy compared to other biological (b) and targeted synthetic (ts) disease-modifying antirheumatic drugs (DMARDs) in achieving ACR70 and PASI90 criteria
What are the new findings?
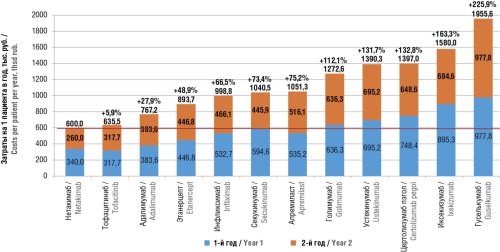
► A 2-year study revealed that other biologic therapy for PsA incurred costs ranging from 6% to 226% higher compared to netakimab. The cost of response achieved with netakimab proves to be minimal compared to other bDMARDs and tsDMARDs
► According to the assessment of potential cost-sharing schemes, achieving equal cost-effectiveness with netakimab requires the cost of comparators to be reduced by more than 50%, except for tofacitinib
How might it impact the clinical practice in the foreseeable future?
► To achieve a comparable cost-effectiveness ratio to that of netakimab based on ACR70 and PASI90 criteria, the reduction in the prices of com-
parators or the provision of additional free packages by the manufacturer are required
► The expansion of using netakimab in PsA treatment is expected to enhance therapeutic options and improve patient prognosis
Objective: to conduct a clinical and economic analysis of the therapy for adult patients with psoriatic arthritis (PsA) using netakimab, a Russian interleukin-17 inhibitor, in comparison with other biological (b) and targeted synthetic (ts) disease-modifying antirheumatic drugs (DMARDs), available on the pharmaceutical market of the Russian Federation.
Material and methods. The evaluation of clinical and economic effectiveness was carried out from the perspective of the healthcare system for a population of patients with active PsA, based on the results of a network meta-analysis. Clinical effectiveness was analyzed through the changes in articular symptoms according to the American College of Rheumatology ACR70 criteria, as well as changes in cutaneous symptoms evaluated by the Psoriasis Area and Severity Index PASI90. A time horizon of the study was set at 2 years. The costs of pharmaceuticals were calculated based on the registered maximum retail prices, inclusive of value-added tax. In case of availability of biosimilars/generics on the market, calculations were based on the minimum values of the registered prices. The impact of price changes on the study results was assessed using sensitivity analysis.
Results. According to the network meta-analysis, the efficacy of netakimab at week 24 significantly surpasses that of adalimumab, apremilast, guselkumab, ixekizumab, secukinumab, tofacitinib, ustekinumab, certolizumab pegol, and etanercept in terms of achieving ACR70; netakimab demonstrated a trend towards higher efficacy in comparison with golimumab and infliximab. The netakimab's efficacy appeared significantly greater than that of adalimumab, golimumab, infliximab, secukinumab, ustekinumab, certolizumab pegol, and etanercept with respect to PASI90 criterion; netakimab exhibited a trend towards higher efficacy in comparison with guselkumab and ixekizumab. The observed clinical effect was accompanied by a significant reduction in healthcare system costs: therapy using comparators over a 2-year study horizon was found to be 6–226% costlier than netakimab-based therapy. The expenditure required to achieve a response based on efficacy criteria was minimal among all evaluated regimens when using netakimab. For comparators, the costs to achieve an additional case of response according to the ACR70 criterion were higher by 127.7–894.6%, and by 59.2–2091.3% according to the PASI90 criterion.
Conclusion. The treatment regimen for PsA with netakimab is characterized by the lowest cost compared to other bDMARDs and tsDMARDs, while providing better or comparable clinical effect, thereby indicating a higher clinical and economic effectiveness. In order to ensure costs comparable to those associated with netakimab and responses based on ACR70 and PASI90 criteria, substantial reductions in prices of comparators or provision of additional free packages by the manufacturer are required. The inclusion of netakimab in the treatment regimens for PsA significantly enhances an accessibility of therapy and contributes to an improved prognosis for disease course.
What is already known about thе subject?
► Calculating the cost of medical care (MC) as a crucial component of the healthcare financing system influences directly the accuracy of funding requirements, the allocation of resources, and consequently, the effectiveness of the government sources used for the health protection of citizens
► As of today, various methodologies for calculating the cost of MC are used in Russia, differing in terms of application area, target audience, level of detail, and calculation characteristics, among other factors. However, they cannot adequately address the current needs of the healthcare system and interests of all its stakeholders, including medical organizations
What are the new findings?
► The analysis of existing methodologies for calculating the cost of MC revealed their significant fragmentation and uncertainty regarding specific interpretations; these methodologies were developed at different times for various objectives by different expert groups
► The developed standardized methodology encompasses all key current principles for calculating the cost of MC (i.e., it is not considered as revolutionary), however, it enhances the transparency and accuracy of calculations, as well as possesses a broad scope of application and can be easily adapted to various objectives
How might it impact the clinical practice in the foreseeable future?
► The calculation of the cost of MC according to a versatile approach is aimed at enhancing the standardization and manageability of healthcare funding at all levels
► The transition to a standardized methodology for calculating the cost of MC will enable more objective calculations, thereby leading to increased accessibility of MC for the population
Background. Current challenges in healthcare include an increase in life expectancy and in medical care (MC) needs, thereby putting pressure on the limited resources of the Russian healthcare system. Efficient allocation of financial resources is becoming a priority. Calculating the cost of MC is considered as a key tool that affects the efficiency of the system. However, the existing calculation methods remain fragmented, which reduces their transparency and limits their application. The need for the unification of approaches to ensure standardization, improve the quality, and increase the accessibility of MC underscores the relevance of the present study.
Objective: to develop a standardized methodology for calculating the cost of MC funded by government sources, aimed at enhancing transparency, standardizing calculations, and supporting a versatile approach in healthcare management.
Material and methods. The study was based on the analysis of regulatory documentation for calculations in the public healthcare sector. The study methodology involved information search techniques, expert surveys, statistical data processing, and economic analysis.
Results. The analysis of existing calculation methodologies revealed their fragmentation, disparities in the level of detail, methods for determining costs, and specific interpretations. The developed standardized methodology included the categorization of costs into direct and indirect, as well as the use of both normative and actual data. The cost of medical services and MC cases were considered the primary calculation units. The methodology was adapted to accommodate various funding sources and regional characteristics.
Conclusion. The suggested methodology enhances the transparency, detail, and consistency of calculations, thereby facilitating effective management of resources within the healthcare system. Flexibility of the approaches allows for further adaptation to meet the needs of different stakeholders within the system.
What is already known about thе subject?
► Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce the level of gastroprotective prostaglandin E2 by inhibiting cyclooxygenase-1, and lower the synthesis of pro-inflammatory prostaglandins by inhibiting cyclooxygenase-2
► The use of zinc-NSAID complexes do not cause significant damage to stomach and intestinal mucous membranes; only mild micro-lesions of gastric mucosa are observed
► The addition of zinc to NSAIDs makes these compositions essential sources of the trace element zinc, characterized by independent anti-inflammatory and immunomodulatory properties
What are the new findings?
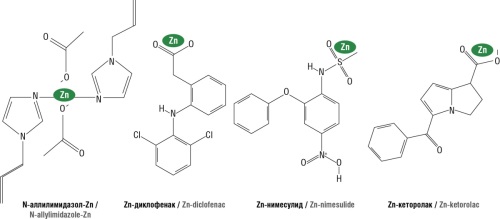
► It was shown that zinc-containing compound N-allylimidazole-zinc (bis-(N-allylimidazole) zinc diacetate) is a promising anti-inflammatory substance, potentially devoid of NSAID disadvantages
► It was determined that anti-inflammatory effect of N-allylimidazole-zinc is due to its effect on cytokine activity and, in part, on prostaglandin and leukotriene metabolism
► The analgesic effect of N-allylimidazole-zinc may be associated with inhibition of kinin receptors, weak antihistamine and antinociceptive effects. N-allylimidazole-zinc may have gastroprotective properties
How might it impact the clinical practice in the foreseeable future?
► With a daily requirement for zinc of about 15–20 mg, N-allylimidazole-zinc and the studied zinc-NSAIDs are significant sources of elemental zinc
► Chemoreactomic analysis of N-allylimidazole-zinc indicates the prospects of creating a drug based on it with pronounced anti-inflammatory, analgesic properties that will not have an ulcerogenic effect
Background. Nonsteroidal anti-inflammatory drugs (NSAIDs) are used for effective and safe pharmacotherapy of inflammation and pain. NSAIDs usually reduce the level of gastroprotective prostaglandin E2 due to cyclooxygenase-1 inhibition. The zinc-containing candidate molecule N-allylimidazole-zinc (bis-(N-allylimidazole) zinc diacetate) is a promising anti-inflammatory drug, potentially devoid of gastrotoxicity.
Objective: chemoreactome modeling of the pharmacological effects of N-allylimidazole-zinc and zinc derivatives of known NSAIDs (diclofenac, nimesulide, ketorolac) using topological analysis of chemographs of numerical prediction in complex feature systems.
Material and methods. In silico modeling of the candidate molecule N-allylimidazole-zinc synthesized at Federal Research Center “Favorsky Irkutsk Institute of Chemistry” (Siberian Branch of Russian Academy of Sciences), was carried out using a conglomerate of chemoinformatic molecule analysis methods of Yu.I. Zhuravlev scientific school. These methods include the theory of chemograph analysis, methods for predicting numerical target variables, combinatorial theory of solvability/regularity, topological data analysis. Chemoreactome, pharmacoinformation and chemoneurocytological methods of analyzing the molecules properties are based on chemoreactome methodology, the latest direction in the application of machine learning systems in the field of postgenomic pharmacology. The pharmacological capabilities of molecules within chemoreactome methodology are assessed by comparing the chemical structure of racetam molecules with the structures of molecules for which the molecular pharmacological properties have been studied using artificial intelligence learning algorithms based on big data information presented in PubChem, HMDB, STRING, PharmGKB databases. Based on the entire complex of differences between molecules in interactions with receptor proteins, the “anti-obesity” score was calculated for each as the serial number of this molecule when sorting in descending order the values of the corresponding chemoreactome constants.
Results. It was shown that N-allylimidazole-zinc may have anti-inflammatory effect due to the influence on cytokine activity and, in part, on prostaglandin and leuktriene metabolism. Its central effects are comparable to the effects of zinc-NSAIDs. The analgesic potential of N-allylimidazole-zinc may be associated with the inhibition of kinin receptors, weak antihistaminic and antinociceptive properties. The molecule may exhibit a protective effect on epithelial gastric mucosa and does not impair the properties of the stomach mucosal protective layer. It has been shown that N-allylimidazole-zinc, compared to other compounds included in the analysis, has the least negative effect on the metabolism of various vitamins and microelements.
Conclusion. Chemoreactome profiling of N-allylimidazole-zinc indicates the prospects for its use as an anti-inflammatory drug.
What is already known about thе subject?
► ABC/VEN analysis is a simple and effective method of pharmacoeconomic research
► Polypragmasy in elderly patients is an actual problem in clinical practice
► Using the Beers criteria enables to optimize pharmacotherapy in elderly patients and prevents the prescription of potentially inappropriate drugs
What are the new findings?
► For the first time, the results of a joint assessment of the rationality of drug procurements using ABC/VEN analysis and comprehensive study by the Beers criteria and the number of cases of polypragmasia in elderly patients were presented on the example of additional preferential drug provision (PDP) providing the optimization of drug prescription and procurement within PDP
How might it impact the clinical practice in the foreseeable future?
► Arranging an annual сomprehensive ABC/VEN analysis in medical institutions enables to rationalize drug procurements
► Rational pharmacotherapy and the use of Beers criteria in medical institutions will ensure decreasing in drug load and improving the safety of drug therapy in elderly patients
Objective: to conduct a pharmacoeconomic evaluation of the rationality of prescribing medications and to analyze the frequency of prescribing potentially inappropriate medications to patients over 65 years old using the example of additional preferential drug provision.
Material and methods. The analysis was based on registers of medications dispensed within the framework of additional drug provision over 2019–2021 period. We conducted an integrated study combining ABC/VEN analysis and the Beers criteria to assess the rationality of pharmacotherapy.
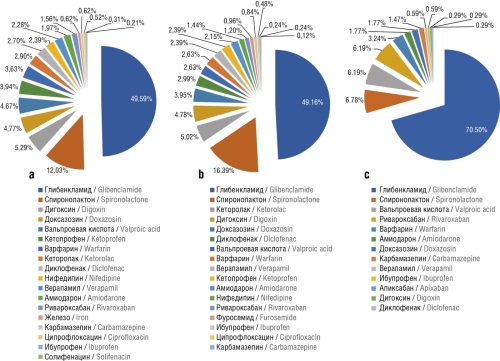
Results. The analysis of the cost structure for medicines showed a positive trend in the distribution of categories V, E, N (vital, essential, nonessential) in A, B, C groups. In 2019, 13.7% of the budget was spent on the procurements of drugs of N category; in 2020, this indicator was 10.6%; and in 2021, the costs for the procurements of drugs of N category decreased to 2.6%. Expenditures for drugs in V and E categories increased from 86.3% to 97.4%. The prevalence of polypragmasy (prescription frequency of 5 or more drugs with different international nonproprietary names per patient for month) among patients over 65 years old reached 11% in 2019, 13% in 2020, and 1% in 2021. According to the Beers criteria, patients over 65 years old were prescribed potentially inappropriate drugs according to the Beers criteria in 12.03% of cases in 2019, in 10.84% of cases in 2020, and in 9.7% of cases in 2021.
Conclusion. Due to comprehensive ABC/VEN analysis and the Beers criteria tool it was possible to evaluate the rationality of expenditures on additional drug provision over the period from 2019 to 2021, as well as the rationality of prescribing medications to patients over 65 years old. The use of Beers criteria enables to optimize pharmacotherapy in elderly patients.
What is already known about thе subject?
► According to epidemiological data, 537 million people aged 20 to 79 years suffer from type 2 diabetes mellitus (T2DM2) worldwide. The number of T2DM patients is expected to increase to 643 million by 2030
► In diabetic polyneuropathy, neuroinflammation leads to demyelination. Schwann cell aging entails myelin loss and impaired axonal function, as well as increases axonal vulnerability to hypoxic or oxidative injuries, and neuroaxonal degeneration
What are the new findings?
► Proteome proteins whose activity were associated with the effects
of adenosine and vitamins B1, PP, B12 were identified
► Target proteins involved in reducing neuroinflammation, remyelination, neurogenesis, neuronal adenosine triphosphate biosynthesis, and myelin homeostasis were identified
How might it impact the clinical practice in the foreseeable future?
► The molecular mechanisms of synergism of the studied molecules are of fundamental importance for comprehension of the regulation processes of neuroinflammation and remyelination to prevent diabetic polyneuropathy and other neurodegenerative diseases
Background. Neurotransmitter adenosine and B-group vitamins have neuroprotective, remyelinizing and anti-neuroinflammatory properties. Despite the studies of these molecules for decades, the molecular mechanisms of their synergistic effect on neuroinflammation processes are unexplored and not systematized.
Objective: to establish the molecular mechanisms of synergism of adenosine, thiamine, niacin and cyanocobalamin in counteracting the pathology of diabetic polyneuropathy (DPN).
Material and methods. The molecular mechanisms of action of adenosine, thiamine (vitamin B1), niacin (vitamin PP) and cyanocobalamin (vitamin B12) in the pathophysiology of DPN were determined using functional analysis of genomic and proteomic databases.
Results. The analysis of 20,180 annotated proteins of the human proteome identified 504 vitamin-PP-dependent, 22 vitamin-B1-dependent, 24 vitamin-B12-dependent and 50 adenosine-dependent proteins. The proteins of the human proteome were detected, the activity or levels of which are important for reducing neuroinflammation, remyelination, neurogenesis, biosynthesis of neuronal adenosine triphosphate, myelin homeostasis, neuroplasticity, neutralization of homocysteine, regeneration of nerve fibers and maintaining the endothelium of the microvascular bed.
Conclusion. The discovered molecular mechanisms of synergism of the studied molecules are of fundamental importance for comprehension of the processes of neuroinflammation regulation and remyelination to prevent diabetic polyneuropathy and other neurodegenerative diseases.
REVIEW ARTICLES
What is already known about thе subject?
► An increasing number of patients with chronic diseases use biologically active supplements (BAS); however, a global consensus regarding their legal regulation remains elusive
► In many countries, studies are still not required when registering BAS to confirm their safety and efficacy due to the alleged “low risk” of natural products
What are the new findings?
► The issues of BAS efficiency and safety were considered, the need for developing an effective strategy for cooperation between consumers, practicing physicians and government institutions, as well as for international criteria for assessing the risks of adverse events was demonstrated
► In Russia, it was proposed to establish a more comprehensive legal regulation of BAS market similar to the control over drugs and medical products, to legislate a mechanism for studying BAS therapeutic and preventive properties using scientific methods
► To harness the full potential of BAS, it is necessary to create a com-
prehensive system of preclinical and clinical studies, actively educate consumers and healthcare professionals about potential drug interactions, and ensure careful adherence to good manufacturing practices by industry representatives
How might it impact the clinical practice in the foreseeable future?
► Strict regulation and effective control system will allow for adequate monitoring and recording of complications that may arise from taking BAS, which will eventually increase their therapeutic effectiveness
► The implementation of the proposed measures will increase transparency and trust in BAS, the competence of healthcare professionals and consumer caution
► Due to the presented list of herbal ingredients with drug interactions doctors will be able to consider BAS when planning treatment strategy
In October 2024, the Dietary Supplement Health and Education Act (DSHEA) of the United States celebrated its 30th anniversary, having established a new class of supplementary medical products. Over the 30-year period, the use of dietary supplements in the United States has evolved from a few hundred products primarily consisting of vitamins, minerals, and select herbal extracts to more than 75,000 items. Despite the popularity of biologically active supplements (BAS), their concurrent use alongside conventional prescription medications raises concerns regarding potential drug interactions, particularly among individuals with comorbidities. An increasing number of patients with chronic diseases use BAS; however, a global consensus regarding their safety remains elusive. The present paper reviews the efficacy and safety of BAS, as well as the market for such products. The presented data underscore the risks associated with the global proliferation of BAS, thus necessitating the development of an effective collaboration among consumers, practicing physicians, and government institutions, as well as the establishment of international criteria for assessing the risks of adverse events. This eventually enhances transparency and trust in the products. In order to ensure safety, a stricter regulation and an efficient control system are required to facilitate adequate monitoring and recording of complications that may arise due to the use of BAS.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
ISSN 2070-4933 (Online)